Array CGH examination
The purpose of this method is to detect small unbalanced rearrangements on chromosomes (losses and gains of genetic material) that cannot be captured by G-banding. Detected rearrangements may cause the patient a serious clinical condition or significantly increase the risk for his/her descendants due to loss (or over-function or lack of function) of affected genes.
The principle of the method is to compare control DNA from a healthy person with the DNA of a tested person. During the procedure both control and tested DNAs are marked with fluorescent dyes, hybridized to microarray chip with high quality probes and than processed for image analysis by Agilent Cytogenomics software. This step is followed by detailed analysis to evaluate the significance of device-marked aberrations and their relevance to individual proband health conditions (or ultrasound findings in the fetus). The final results are described with regards to the worldwide medical databases and academic literature with the same or similar findings, thus refining the outcome of the genetic consultation performed by a physician in our center.
The disadvantage of this method is its high sensitivity to the quality of the input material and it does not detect chromosome aberrations without gain or loss of genetic material. Therefore it cannot be used to detect clinically healthy carriers of small balanced chromosomal rearrangements who have a high risk of repeated pregnancy loss or childbirth of a disabled child.
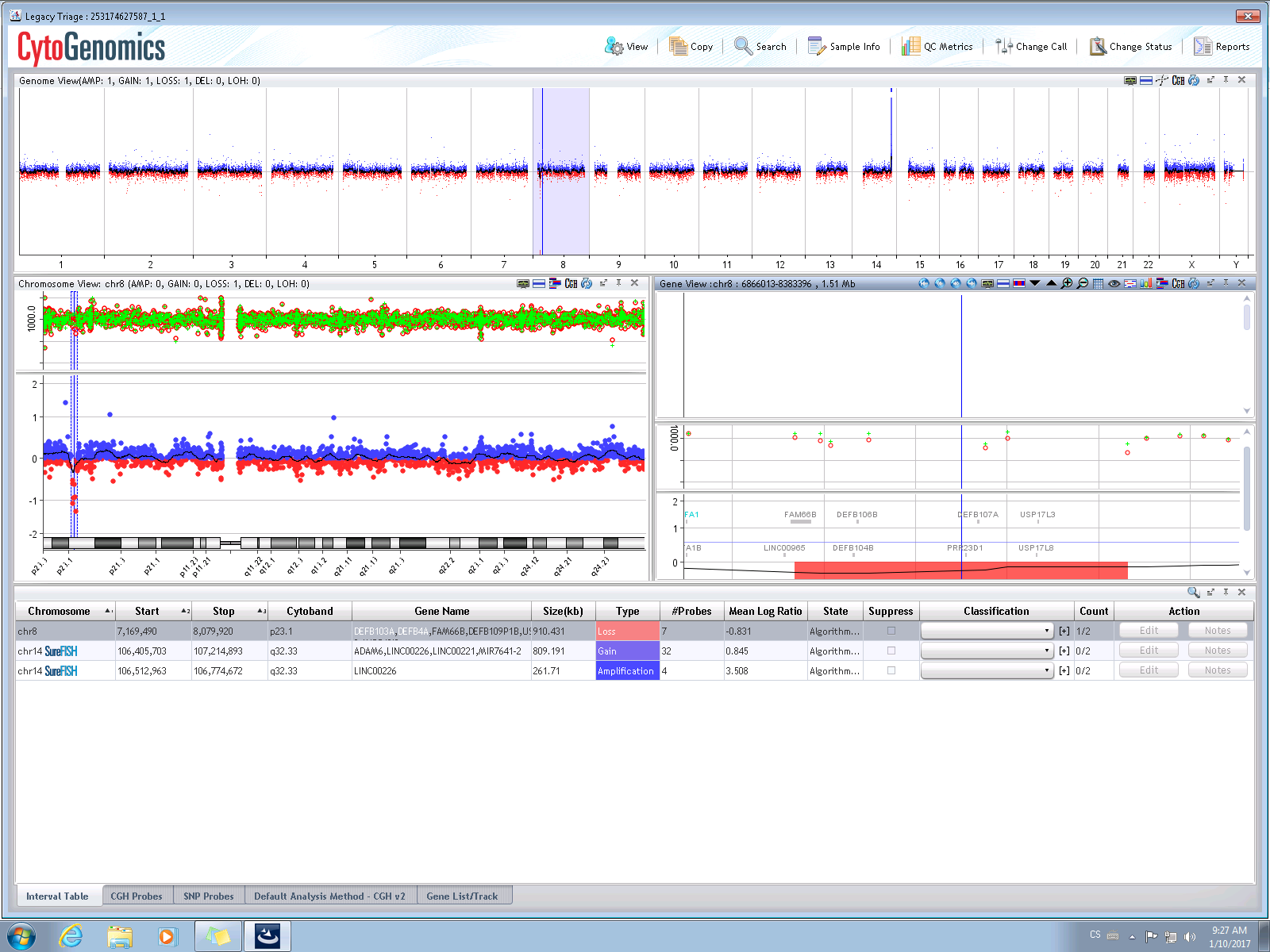
Sample computer analysis output – normal male profile
Karyotypization – chromosomal screening
Karyotypization is a cytogenetic examination used to determine the chromosomal set of the individual. We can examine peripheral blood cells, amniotic fluid, and the chorionic villus or fetal tissue of a miscarriage as needed.
The biological material is cultivated under sterile conditions in a nutrient medium in order to contain a large number of dividing cells. The grown cell suspension is then used as a sample on a glass slide and stained. The number and structure of chromosomes in dividing cells can be evaluated under a microscope. The complete karyotype can be determined after examining the sufficient number of cells.
The human karyotype has 46 chromosomes; 22 pairs (one set from each parent) and two sex chromosomes: two X chromosomes in a female and X and Y chromosomes in a male. Each chromosome has a specific shape and a certain sequence of light and dark bands (G bands). The chromosomes are evaluated according to the international system of cytogenetic nomenclature ISCN. Variations in the structure or number of chromosomes are considered pathological findings.
Our laboratory is accredited according to ČSN EN ISO 15189.
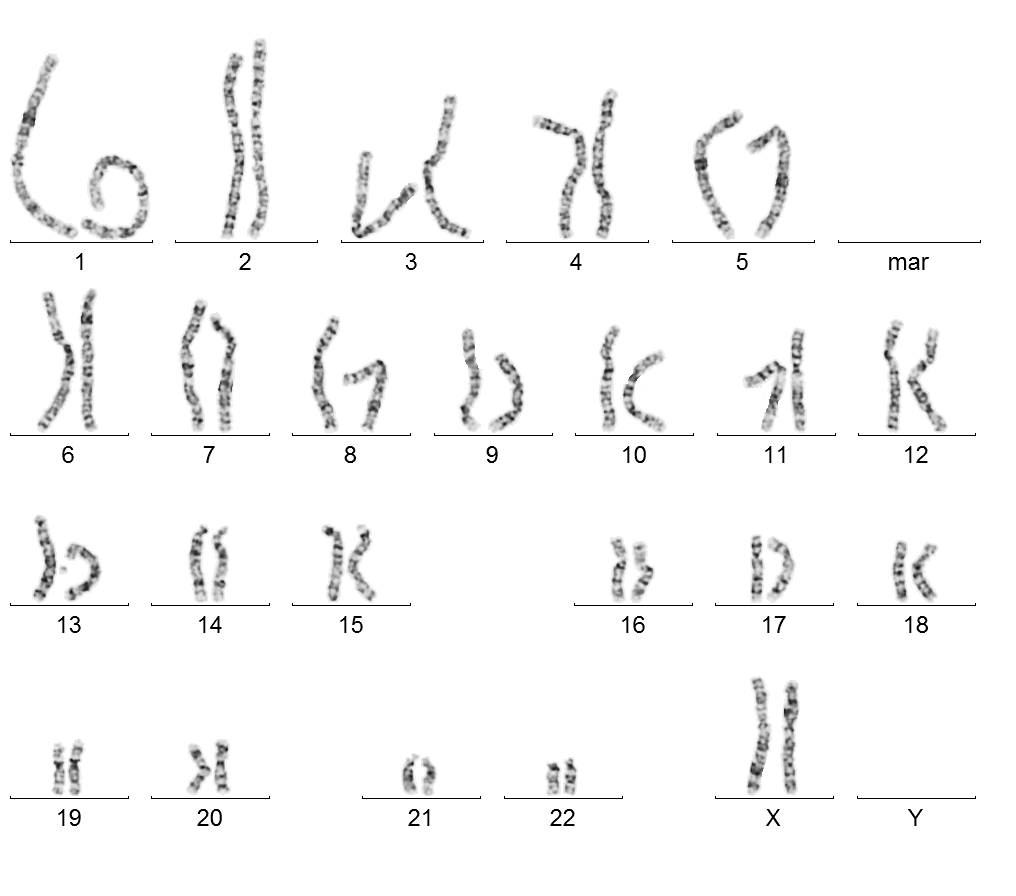
Female karyotype 46, XX

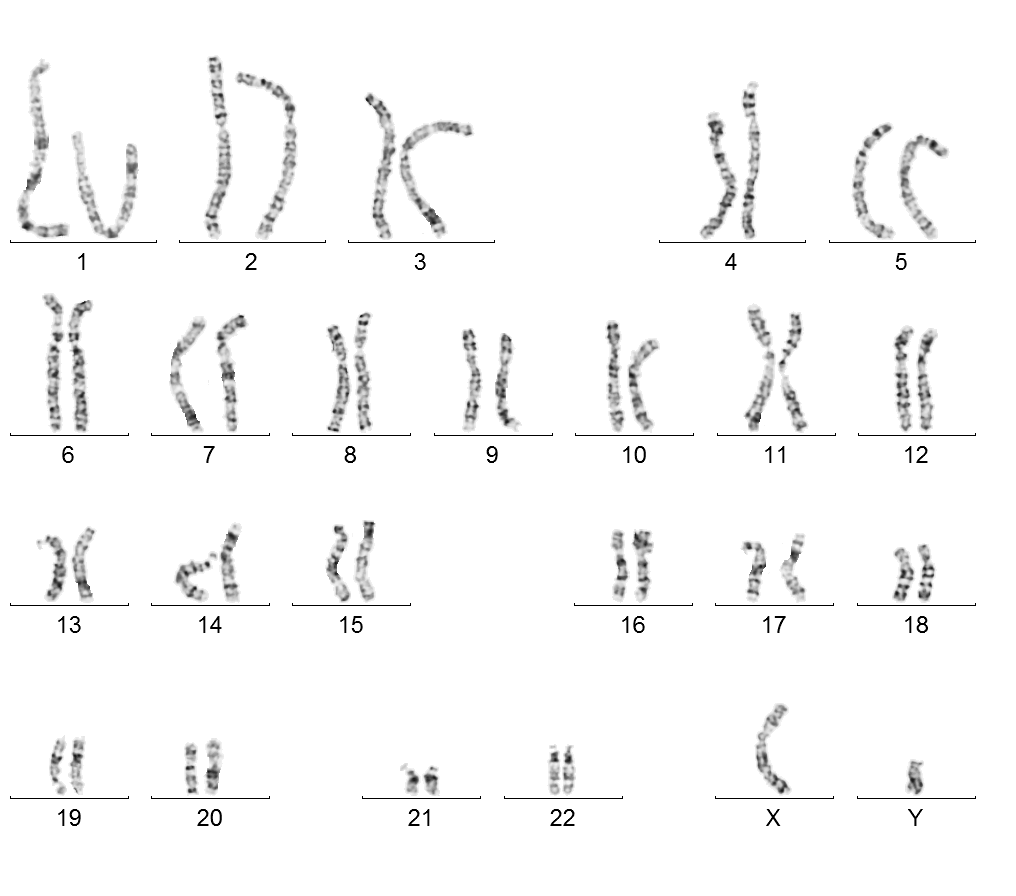
Male karyotype 46, XY
Examination using the FISH method
FISH screening in our laboratory is used to determine and specify the variations in the number and structure of chromosomes in biological material – specifically and primarily in blood and amniotic water.
FISH, or Fluorescence In Situ Hybridization, utilizes artificially prepared color-coded DNA segments, known as probes, that are bound to the corresponding portions of the chromosomal DNA of the examined sample. Hybridization takes place in situ, or “on site”, specifically on a microscopic backing slide directly on the cores of the examined sample. The result shows the presence or absence of whole chromosomes, or certain segments of DNA, the number of copies of these sections, and sometimes even their locations on the chromosomes. The presence of the examined section is visible in color in a fluorescent microscope thanks to the bound and marked probe.
A sample of the translocation (transfer) of part of chromosomes 16 and 7 (in the latter case, chromosomes 12 and 18) to form derived chromosomes: the examined material in these cases is peripheral blood.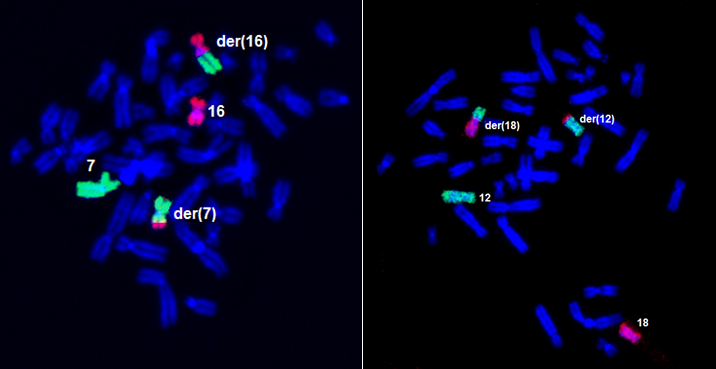
A sample routine examination of a numerical aberration of chromosomes X, Y , 13, 18, 21: the examined material is amniotic water.
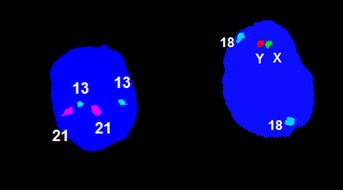
Examination by QF PCR method
Molecular genetic examination (QF PCR) is a method performed in patients at high risk of fetal chromosomal abnormalities (older age, positive biochemical screening, ultrasound fetal abnormalities, etc.).
The fetal DNA is obtained from chorionic villus collection (CVS) or amniotic fluid (AC). The QF-PCR method (quantitative fluorescent PCR) allows for rapid prenatal detection of aneuploidies for chromosomes, most frequently 13, 18, 21, X and Y. The polymerase reaction with fluorescently labeled primers allows for the separation and quantification of specific markers on an automated genetic analyzer. The mother’s DNA is also analyzed for comparison.
The advantage of this examination is its speed and accuracy (results can be processed by the next working day).







